New grant awarded by the Francis Crick institute to Dr Ellen Knuepfer
Dr Ellen Knuepfer was awarded a grant by The Francis Crick Institute to carry out research on the Characterisation of novel protein complexes which play an essential role in erythrocyte invasion by Plasmodium and Babesia parasites and to investigate their vaccine potential.
Plasmodium and Babesia are haemoparasites of vertebrates. Plasmodium is the causative agent of malaria and transmitted by mosquito bite. World-wide more than 200 million malaria cases are reported annually, resulting in more than 400,000 deaths, predominantly in young children in sub-Saharan Africa. Whereas the dominant Plasmodium parasite in Africa is restricted to infect humans, a zoonotic Plasmodium species in Asia infects both macaques and humans posing a significant human disease burden in countries such as Malaysia. The only vaccine currently available has very limited efficacy and is directed against the Plasmodium species dominant in Africa.
Babesia parasites, like Plasmodium, live intracellularly in erythrocytes and cause a vector-borne disease very similar to malaria. Veterinary-relevant Babesia species infect cattle, small ruminants, as well as dogs. Some Babesia spp can cause zoonotic disease affecting humans, such as B.divergens and B.venatorum, which are endemic in the UK. Also recently introduced to the UK was B.canis, which can cause severe disease in dogs. Symptoms of babesiosis include high fever, haemoglobinuria, anaemia, and can include neurological signs.
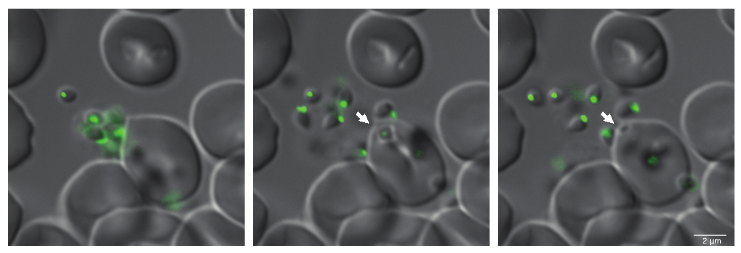
Studying conserved, secreted proteins in different Plasmodium species and their role during erythrocyte invasion by generating inducible knockout parasites using CRISPR-Cas9 tools, we have identified a protein complex which appears essential for successful host-cell invasion of the parasite. We have also shown that antibodies targeting components of this complex are effective in blocking host cell invasion. We seek to address whether this novel protein complex is required for host cell invasion of all human malaria-causing parasites, and whether antibodies targeting this complex could function as a pan-Plasmodium vaccine.
By detailed analyses of steps involved in erythrocyte invasion by Plasmodium and Babesia parasites using transgenic parasite lines, we aim to identify proteins involved in crucial steps during this process, such as proteins involved in host cell recognition and signal transduction for coordinated release of invasion-related organelles which will allow us to better understand parallels in the overall conserved mechanisms of erythrocyte invasion by parasites of these two related genera.