

The International Feline Encephalitis Study Group - Autoimmune encephalitis in cats and humans
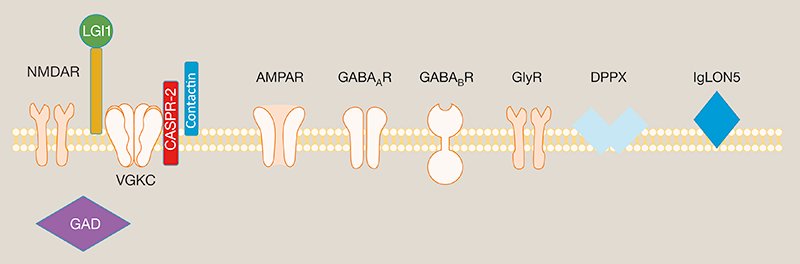
In the past ~15 years, it is increasingly recognised that a number of neuronal cell surface proteins can be targets for pathogenic autoantibodies. These autoantibodies bind to their target in vivo and disrupt normal brain functioning, causing clinical presentations including seizures and limbic encephalitis (LE).
LGI1-autoantibodies in humans
One target protein in autoimmune encephalitides is leucine-rich glioma-inactivated 1 (LGI1), a secreted molecule which acts a synaptic bridge and is particularly richly expressed in the hippocampus. Human patients with LGI1-autoantibodies typically present with a LE of sub-acute onset. A hallmark of this type of encephalitis are faciobrachial dystonic seizures (FBDS), a brief episode causing uni- and occasionally bilateral jerking of the arm, face and/or leg. Other common features in the acute phase include frequent and varied focal seizures, amnesia, personality change and behavioural disturbance. Human patients with LGI1-antibodies typically respond very well to immunotherapies, including corticosteroids and plasma exchange.
LGI1-autoantibodies in domestic cats
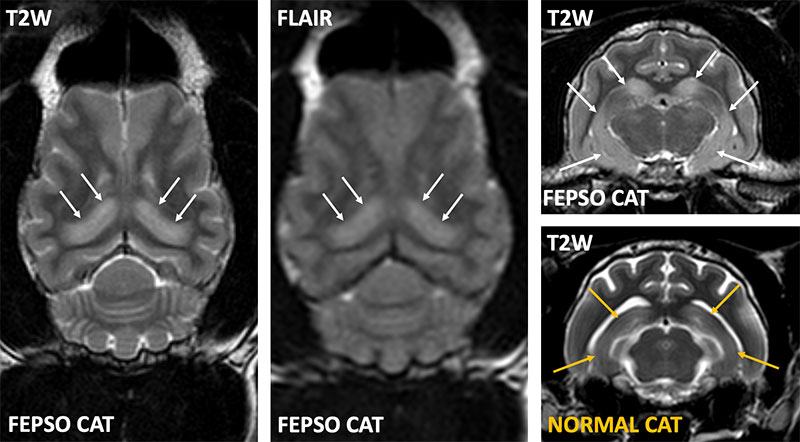
Recently, it was described that some cats with Feline Partial Cluster Seizures with Orofacial Involvement (FEPSO) have LGI1-autoantibodies. The seizures are typically focal with orofacial automatisms (licking, chewing, lip smacking, hypersalivation), altered consciousness, mydriasis and vocalisation. Serological, clinical, MRI and histopathological features of these cats demonstrate remarkable similarities to their human counterparts. Optimal management for these cats is not fully understood and euthanasia may even be considered for refractory feline cases. However, many cats have a milder disease course and even severely affected cats can do well with prompt recognition and treatment.
Our aim is to study these cats in greater detail with the ultimate goal of improving diagnosis and outcomes in FEPSO, translating advances from human to feline medicine, and vice versa, in a bidirectional translational model.
*These include the NMDAR (N-methyl-d-aspartate receptor), AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor), LGI1 (leucine-rich glioma-inactivated protein 1), CASPR2 (contactin-associated protein 2), GAD (glutamic acid decarboxylase), GABAᴀ or GABAʙ receptors (γ-aminobutyric acid), GlyR (glycine receptor), DPPX (dipeptidyl-peptidase-like protein 6) and IgLON5 (Ig-like domain-containing protein 5).
