Dr Matthew Gage
Department: Comparative Biomedical Sciences
Campus: Camden
Research Groups: Cardiovascular and Renal Biology, CPCS (Research Programme), Brain Health and Behaviour
Dr Gage is a Lecturer and Research Group Leader at the Royal Veterinary College.
His research group is interested in how insulin affects inflammation and the function of the immune system, and how in turn this may impact the development and treatment of diabetes and cardiovascular disease.
Dr Gage's group is gratefully funded by the British Heart Foundation, Diabetes UK and the Society for Endocronology.
Dr Matthew Gage completed his PhD at Leeds University (UK) investigating cell signalling and secretion in the inflammatory cell type: mast cells. Matthew then went on to work in the Division of Cardiovascular and Diabetes Research also at Leeds University, before moving on to the Division of Medicine at University College London (UK) to investigate inflammatory mechanisms in macrophages. Dr Gage now leads a research group at the Royal Veterinary College, investigating inflammation in diabetes and cardiovascular disease.
Professional Activities
Review Editor: Frontiers in Cardiovascular Medicine 2023 - present https://www.frontiersin.org/journals/cardiovascular-medicine, https://loop.frontiersin.org/people/1365777/overview
Corpoate Liason Committee member: Society for Endocroinology 2022-2026 https://www.endocrinology.org/
Scientifc Committee member: Society for Endocrinology 2022-2026 https://www.endocrinology.org/
Gene Ontology Consortium contributor: http://geneontology.org/docs/whoweare/
Honorary Lectureship: Centre for Cardiometabolic & Vascular Science, Division of Medicine, University College London https://www.ucl.ac.uk/cardiovascular-biology/
Committee Member: London Vascular Biology Forum https://www.kcl.ac.uk/scms/lvbf
2019-2022 Diabetes UK: ‘IDia – Innovators in Diabetes’ Programme participant https://www.diabetes.org.uk/
2017-2019 Volume Editor: Methods in Molecular Biology: Lipid Activated Nuclear Receptors (vol 1951), Springer Nature, 2019
2017 – 2019: UCL Division of Medicine BHF Fundraising group coordinator.
2017 - 2019: Metabolism & Society @UCL: Academic Lead for Early Career Research Involvement
Recent Awards:
2018 BHF Researcher Engagement Award, Group Finalist.
2016 UCL Cardiovascular Symposium, 1st Prize winner.
2014 Diabetes UK, Nick Hales Award - Young Investigator Finalist
2012 British Cardiovascular Society - Young Research Workers' Prize Runner-up
2011 Diabetes UK, Nick Hales Award - Young Investigator Finalist.
2009 British Atherosclerosis Society - Young Investigator Finalist.
Previous posts
2016 - 2019: British Heart Foundation Principal Investigator, Division of Medicine, University College London, UK
2013 - 2016: Senior Research Associate, Division of Medicine, University College London, UK
2008 - 2013: Postdoctoral Research Fellow, Division of Cardiovascular & Diabetes Research, University of Leeds, UK
2002 - 2006: PhD Dept of Biochemistry, University of Leeds, UK
1998 - 2002: BSc Int (Hons) Biological Sciences with Molecular Genetics with Intercalated Year, University of Warwick, UK
Our research is revealing new roles of insulin which may benefit people with diabetes and cardiovascular disease
Insulin is widely recognised for its role in reducing blood glucose levels, however it has recently been shown by our research group and others, that insulin plays a role in inflammation. This dual function may have evolved to protect us from contaminated foods which until recently in human history, we would have been frequently exposed to.
The mechanism of how insulin affects blood glucose has been well studied, but how insulin regulates inflammation is less understood because it is such a recent finding.
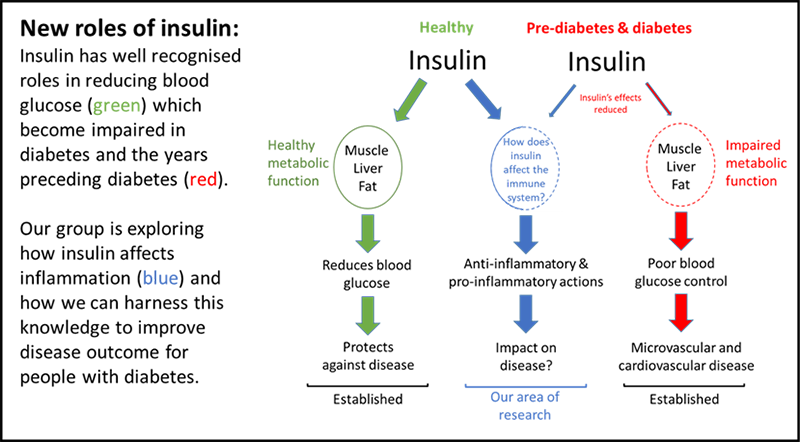
It is important to find out how insulin effects inflammation because insulin levels are significantly higher in the years preceding diabetes diagnosis compared to healthy people; this may contribute to the increased risk of inflammatory diseases such as cardiovascular disease, that people with diabetes suffer from.
Full publication list available at: https://scholar.google.co.uk/citations?hl=en&user=KBNr95MAAAAJ
...and find out more about my publications here: https://www.growkudos.com/profile/matthew_gage
Original research articles
Arefin A, Gage MC. Metformin, Empagliflozin, and Their Combination Modulate Ex-Vivo Macrophage Inflammatory Gene Expression. Int J Mol Sci. 2023;24(5):4785. Published 2023 Mar 1. doi:10.3390/ijms24054785
Gene Ontology Consortium, Aleksander SA, Balhoff J, et al. The Gene Ontology knowledgebase in 2023. Genetics. 2023;224(1):iyad031. doi:10.1093/genetics/iyad031
Becares N, Gage MC, Voisin M, Shrestha E, Martin-Gutierrez L, Liang N, Louie R, Pourcet B, Pello OM, Luong TV, Goñi S, Pichardo-Almarza C, Røberg-Larsen H, Diaz-Zuccarini V, Steffensen KR, O'Brien A, Garabedian MJ, Rombouts K, Treuter E, Pineda-Torra I. Impaired LXRα Phosphorylation Attenuates Progression of Fatty Liver Disease. Cell Rep. 2019 Jan 22;26(4):984-995.e6. doi: 10.1016/j.celrep.2018.12.094.
Gage MC, Bécares-Salles N, Louie R, Waddington K.E, Zhang Yu, Tittanegro T, Rodriguez-Lorenzo S, Jathanna A, Pourcet B, Pello O, De la Rosa JV, Castrillo A and Pineda-Torra I. Disrupting myeloid-specific LXRα phosphorylation promotes FoxM1 expression and modulates atherosclerosis by inducing macrophage proliferation. Proc Natl Acad Sci U S A. 2018 Jun 27. pii: 201721245. doi: 10.1073/pnas.1721245115.
Gage MC*, Watt N*, Viswambharan H, Sukumar P, Imrie H, Galloway S, Yuldasheva N, Smith J, Skromna, Grant PJ, Gatenby V, Beech DJ, Schurmans S, Wheatcroft SB, Kearney MT, Cubbon R. Endothelial SHIP2 suppresses Nox2 NADPH oxidase-dependent vascular oxidative stress, endothelial dysfunction and systemic insulin resistance. Diabetes. 2017 Nov;66(11):2808-2821. doi: 10.2337/db17-0062.
Micochova P, Sutherland KA, Watters SA, Bertoli C, de Bruin RA, Rehwinkel J, Neil SJ, Lenzi GM, Kim B, Khwaja A, Gage MC, Georgiou C, Chittka A, Yona S, Noursadeghi M, Towers GJ, Gupta RK. A G1-like state allows HIV-1 to bypass SAMHD1 restriction in macrophages. EMBO J. 2017 Jan 25. pii: e201696025. doi: 10.15252/embj.201696025.
Viswambharan H, Yuldasheva NY, Sengupta A, Imrie H, Gage MC, Haywood NJ, Walker AM, Skromna A, Makova N, Galloway SL, Shah P, Sukumar P, Porter KE, Grant PJ, Shah AM, Santos CX, Li J, Beech DJ, Wheatcroft S, Cubbon RM, Kearney MT. Selective Enhancement of Insulin Sensitivity in the Endothelium In Vivo Reveals a Novel Proatherosclerotic Signalling Loop. Circ Res. 2016 Dec 5. pii: CIRCRESAHA.116.309678.
Gage MC*, Pourcet B*, Pello OM, Leon TL, Castrillo A, Valledor AF, Pineda-Torra I. The nuclear receptor LXR modulates interleukin-18 levels through multiple mechanisms. Sci Rep. 2016 May 6;6:25481.
Latham AM, Kankanala J, Fearnley GW, Gage MC, Kearney MT, Homer-Vanniasinkam S, Wheatcroft SB, Fishwick CW, Ponnambalam S. In silico design and biological evaluation of a dual specificity kinase inhibitor targeting cell cycle progression and angiogenesis. PLoS One. 2014 Nov 13;9(11)
Yuldasheva NY, Rashid ST, Haywood NJ, Cordell P, Mughal R, Viswambharan H, Imrie H, Sukumar P, Cubbon RM, Aziz A, Gage M, Mbonye KA, Smith J, Galloway S, Skromna A, Scott DJ, Kearney MT, Wheatcroft SB. Haploinsufficiency of the insulin-like growth factor-1 receptor enhances endothelial repair and favorably modifies angiogenic progenitor cell phenotype. Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):2051-8
Cubbon RM, Yuldasheva NY, Viswambharan H, Mercer BN, Baliga V, Stephen SL, Askham J, Sukumar P, Skromna A, Mughal RS, Walker AM, Bruns A, Bailey MA, Galloway S, Imrie H, Gage MC, Rakobowchuk M, Li J, Porter KE, Ponnambalam S, Wheatcroft SB, Beech DJ, Kearney MT. Restoring Akt1 activity in outgrowth endothelial cells from South Asian men rescues vascular reparative potential. Stem Cells. 2014 Oct;32(10):2714-23.
Gage MC, Yuldasheva NY, Viswambharan H, Sukumar P, Cubbon RM, Galloway S, Imrie H, Skromna A, Smith J, Jackson CL, Kearney MT, Wheatcroft SB. Endothelium-specific insulin resistance leads to accelerated atherosclerosis in areas with disturbed flow patterns: a role for reactive oxygen species. Atherosclerosis. 2013 Sep;230(1):131-9.
Sukumar P, Viswambharan H, Imrie H, Cubbon RM, Yuldasheva N, Gage M, Galloway S, Skromna A, Kandavelu P, Santos CX, Gatenby VK, Smith J, Beech DJ, Wheatcroft SB, Channon KM, Shah AM, Kearney MT. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes. 2013 Jun;62(6):2130-4.
Imrie H, Viswambharan H, Sukumar P, Abbas A, Cubbon RM, Yuldasheva N, Gage M, Smith J, Galloway S, Skromna A, Rashid ST, Futers TS, Xuan S, Gatenby VK, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. Novel role of the IGF-1 receptor in endothelial function and repair: studies in endothelium-targeted IGF-1 receptor transgenic mice. Diabetes. 2012 Sep;61(9):2359-68.
Rajwani A, Ezzat V, Smith J, Yuldasheva NY, Duncan ER, Gage M, Cubbon RM, Kahn MB, Imrie H, Abbas A, Viswambharan H, Aziz A, Sukumar P, Vidal-Puig A, Sethi JK, Xuan S, Shah AM, Grant PJ, Porter KE, Kearney MT, Wheatcroft SB. Increasing circulating IGFBP1 levels improves insulin sensitivity, promotes nitric oxide production, lowers blood pressure, and protects against atherosclerosis. Diabetes. 2012 Apr;61(4):915-24.
Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon RM, Gage M, Smith J, Galloway S, Yuldeshava N, Kahn M, Xuan S, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes. 2011 Aug;60(8):2169-78.
Kahn MB, Yuldasheva NY, Cubbon RM, Smith J, Rashid ST, Viswambharan H, Imrie H, Abbas A, Rajwani A, Aziz A, Baliga V, Sukumar P, Gage M, Kearney MT, Wheatcroft SB. Insulin resistance impairs circulating angiogenic progenitor cell function and delays endothelial regeneration. Diabetes. 2011 Apr;60(4):1295-303.
Imrie H, Abbas A, Viswambharan H, Rajwani A, Cubbon RM, Gage M, Kahn M, Ezzat VA, Duncan ER, Grant PJ, Ajjan R, Wheatcroft SB, Kearney MT. Vascular insulin-like growth factor-I resistance and diet-induced obesity. Endocrinology. 2009 Oct;150(10):4575-82.
Gage MC, Keen JN, Buxton AT, Bedi MK, Findlay JB. Proteomic analysis of IgE-mediated secretion by LAD2 mast cells. J Proteome Res. 2009 Aug;8(8):4116-25.
Breton J, Gage MC, Hay AW, Keen JN, Wild CP, Donnellan C, Findlay JB, Hardie LJ. Proteomic screening of a cell line model of esophageal carcinogenesis identifies cathepsin D and aldo-keto reductase 1C2 and 1B10 dysregulation in Barrett's esophagus and esophageal adenocarcinoma. J Proteome Res. 2008 May;7(5):1953-62.
Letters
Gage MC, Harrington D, Brierley GV, Freathy RM, Gabriel BM, Gibson R, McNeilly AD, Meek CL, Roberts LD. Challenges and solutions for diabetes early career researchers in the COVID-19 recovery: Perspectives of the Diabetes UK Innovators in Diabetes. Diabet Med. 2021 Sep 25:e14698. doi: 10.1111/dme.14698. PMID: 34562338.
Reviews
Sheridan A, Wheeler-Jones CPD, Gage MC. The Immunomodulatory Effects of Statins on Macrophages. Immuno. 2022; 2(2):317-343. https://doi.org/10.3390/immuno2020021
Batty MJ, Chabrier G, Sheridan A, Gage MC. Metabolic Hormones Modulate Macrophage Inflammatory Responses. Cancers. 2021 Sep 17;13(18):4661. doi: 10.3390/cancers13184661. PMID: 34572888; PMCID: PMC8467249.
Thibaut R, Gage MC, Pineda-Torra I, Chabrier G, Venteclef N, Alzaid F. Liver macrophages and inflammation in physiology and physiopathology of non-alcoholic fatty liver disease. FEBS J. 2021 Apr 15. doi: 10.1111/febs.15877.
Gage MC*, Voisin M*, Becares N, Shrestha E, Fisher EA, Pineda-Torra I, Garabedian MJ.
LXRα Phosphorylation in Cardiometabolic Disease: Insight From Mouse Models. Endocrinology, Volume 161, Issue 7, July 2020.
Becares N, Gage MC, Pineda-Torra I. Post-translational modifications of lipid-activated nuclear receptors: Focus on metabolism. Endocrinology. 2017 Feb 1;158(2):213-225. doi: 10.1210/en.2016-1577.
Books edited
Gage MC & Pineda-Torra I (2019) 'Lipid-activated Nuclear Receptors' Springer Nature: Methods in Molecular Biology book series (MIMB, volume 1951).
Book chapters
Gage MC, ‘Measuring Apoptotic Cell Engulfment (Efferocytosis) Efficiency’ for ‘Lipid-activated Nuclear Receptors, Springer. Methods Mol Biol. 2019;1951:143-152. doi: 10.1007/978-1-4939-9130-3_11.
Gage MC, Pourcet B, Pineda-Torra I, ‘Luciferase Reporter Assays to Measure Liver X Receptor Transcriptional Activity’ for ‘Methods in Molecular Biology, Protein Blotting and Detection. Methods Mol Biol. 2016;1376:77-85.
Pineda-Torra I, Gage M, Juan A, Pello OM, ‘Isolation, culture and polarization of murine bone marrow-derived and peritoneal macrophages’ for ‘Methods in Mouse Atherosclerosis’. Methods Mol Biol. 2015;1339:101-9.
Preprints
Aged insulin resistant macrophages reveal dysregulated cholesterol biosynthesis, a pro-inflammatory profile and reduced foam cell formation capacity. G Chabrier, S Hobson, N Yuldasheva, M. T Kearney, S Schurmans, I Pineda-Torra, M. C Gage. doi: https://doi.org/10.1101/467118
Metformin, empagliflozin and their combination modulate ex-vivo macrophage inflammatory gene expression. Adittya Arefin, Matthew C Gage doi: https://doi.org/10.1101/2022.06.20.496771
Dr Matthew Gage delivers biochemistry lectures in cell signallling, metabolism, endocrinology and ageing and further lecures on animal models of diabetes.
Matthew is a tutor to Gateway and BVetMed students.
Public/ patient talks
Matthew regularly presents his research to the public and enjoys talking about 'working in life science', including presenting to comprehensive schools, Women's Institute talks and BHF and Diabetes UK fundraising volunteer groups.
Work experience
Dr Gage's lab also hosts a limited number of school students each summer for work experience in an academic laboratory.
School student summary of Dr. Gage's research:
"The aim of Dr Gage's research is to expand our understanding of what insulin does and how it does it; to help people with diabetes. Insulin affects inflammation - it seems to be able to both promote and reduce inflammation. In a healthy person, insulin levels increase for a short while after we have eaten, before returning to a low level. In the years preceding type 2 diabetes, insulin levels in the blood are higher than normal and increase much more dramatically after we have eaten. If we can determine how a healthy amount of insulin affects the immune system in a good way, we can use insulin more effectively to manage inflammation in diabetes".
- E.H, Ousedale School, 2019



